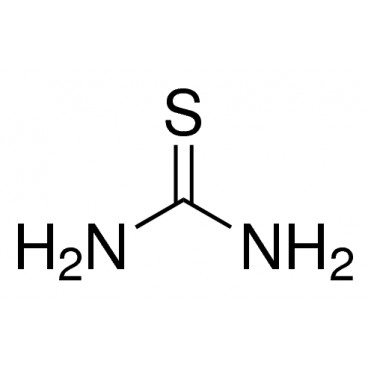
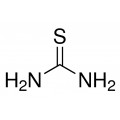
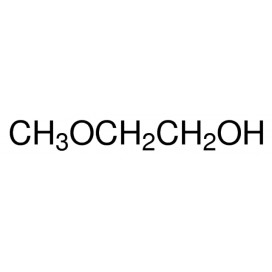
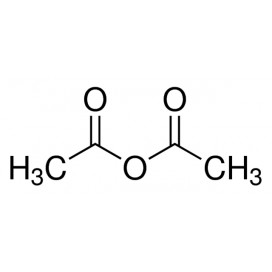
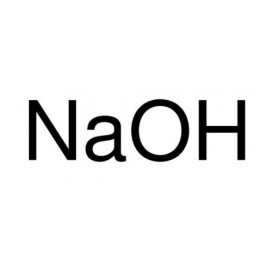
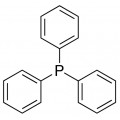
Thiourea organosulfur compound with the formula SC(NH2)2 . It is structurally similar to urea, except that the oxygen atom is replaced by a sulfur atom, but the properties of urea and thiourea differ significantly.
Thiourea is a reagent in organic synthesis.
Thiourea reduces peroxides to the corresponding diols.
Thiourea is also used in the reductive workup of ozonolysis to give carbonyl compounds. Thiourea is commonly employed as a source of sulfide, e.g. for converting alkyl halides to thiols. Such reactions proceed via the intermediacy of isothiuronium salts. The reaction capitalizes on the high nucleophilicity of the sulfur center and easy hydrolysis of the intermediate isothiouronium salt.
Thioureas are used a building blocks to pyrimidine derivatives. Thus thioureas condense with β-dicarbonyl compounds. The amino group on the thiourea initially condenses with a carbonyl, followed by cyclization and tautomerization. Desulfurization delivers the pyrimidine.
Substituted thioureas are useful catalysts for organic synthesis. The phenomenon is called thiourea organocatalysis.
According to the label on the consumer product, the liquid silver cleaning product TarnX contains thiourea, a detergent, and sulfamic acid. A lixiviant for gold and silver leaching can be created by selectively oxidizing thiourea, bypassing the steps of cyanide use and smelting.
The main application of thiourea is in textile processing.
Other industrial uses of thiourea include production of flame retardant resins, and vulcanization accelerators.
Thiourea is used as an auxiliary agent in diazo paper, light-sensitive photocopy paper and almost all other types of copy paper.
It is also used to tone silver-gelatin photographic prints.
Thiourea is used in the Clifton-Phillips and Beaver bright and semi-bright electroplating processes. It is also used in a solution with tin(II) chloride as an electroless tin plating solution for copper printed circuit boards.